
We exist to take care of people and strengthen health. We are committed to putting this objective into practice in the care for our patients every single day. It is already more than 45 years of history and, through all this time, we have experienced many remarkable changes. We grew, developed ourselves and have become one of the best hospitals in the country. But our essence remains the same, turned into an intimate relationship of welcoming and dedicating yourself tirelessly to those who need the most. Because what you feel, for us, makes all the difference.
We are a philanthropic organization and, through the Beneficente Síria Association, we share our care and experience in initiatives that we are proud of. In partnership with the Department of Health, we help SUS (public health system) with assistance, educational and research projects in all Brazilian states. In Sao Paulo city, we offer services to communities on three main pillars : Intrauterine Intervention, Congenital heart disease and supporting refugees.
We became a multi-specialist hospital to offer the better care in important segments, such as cardiology, oncology, orthopedics, neurology and diagnostic medicine.


The Hcor Emergency Service is open 24 hours a day, seven days a week. Our team is formed 100% by specialized doctors.
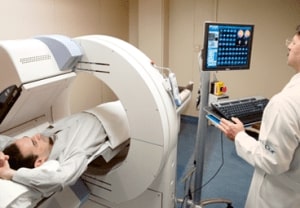
The Hcor Diagnostic Center provides services such as computed tomography, magnetic resonance, ergometry, nuclear medicine, holter, etc.
Home collection of material for laboratory analysis, performed by a nursing professional from Hcor.
Hcor provides the Ombudsman Service, which is the communication channel between hospital areas and clients and their families.
For criticisms, suggestions or compliments, fill out the form below to get in touch with the Hcor team.
Opening hours are from 8:30 am to 7:00 pm, Monday to Friday.